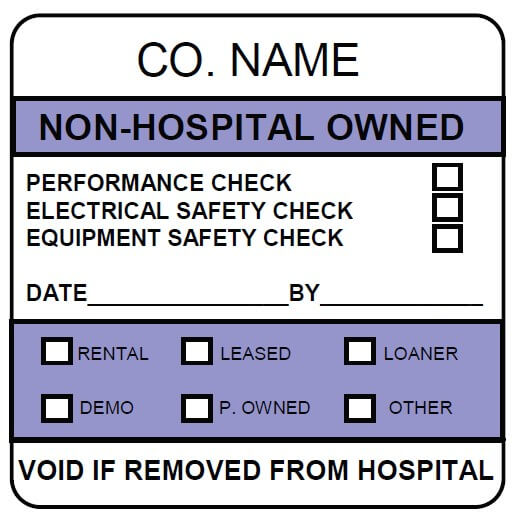
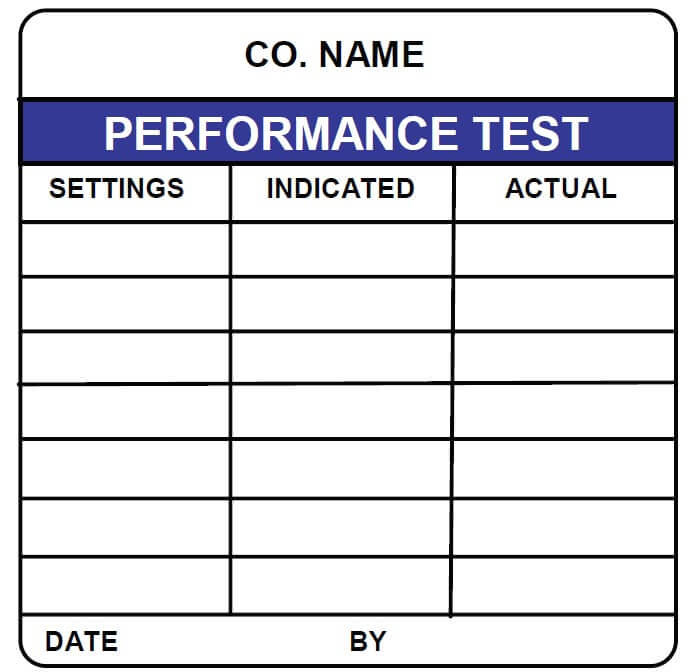
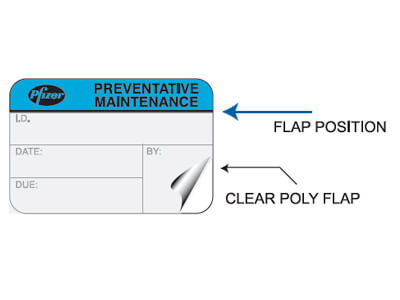
At ID Label, we use the best combination of materials to produce premium-quality barcode labels engineered for your specific application.
ID Label’s turnkey labeling solutions include custom calibration label design and consultation at no extra cost. Through our 20-step quality inspection process, barcodes are verified to ensure 100 percent scanning accuracy.
Our high-quality materials and adhesives and calibration label designs have been proven to perform in hospital, biomedical and clinical settings year after year.