5 Calibration Label Requirements to Help Meet Quality Certification Standards
ISO National Standards
Quality certification standards are vital for many organizations. They help to:
- Improve the quality of products and services
- Minimize mistakes
- Enhance reporting and communications
- Improve operational consistencies and efficiencies
There are numerous quality standards across many industries. ID Label calibration labeling products can help you satisfy virtually all worldwide acceptable quality standards necessary for ISO 9000, 21CFR211, 21CFR820,ISO/IEC Guide 25, QS 9000, ISO14000, ANSI/ASQC Q9001, ANSI/NCSL Z540-1 and OSHA 1910.
This post is a quick primer to help you understand how labels can help you meet and monitor your adherence to the quality standards you are striving for.

 To meet certification standards, labels and tags require direct traceability and must reference several items specific to the equipment and its maintenance and calibration.
To meet certification standards, labels and tags require direct traceability and must reference several items specific to the equipment and its maintenance and calibration.
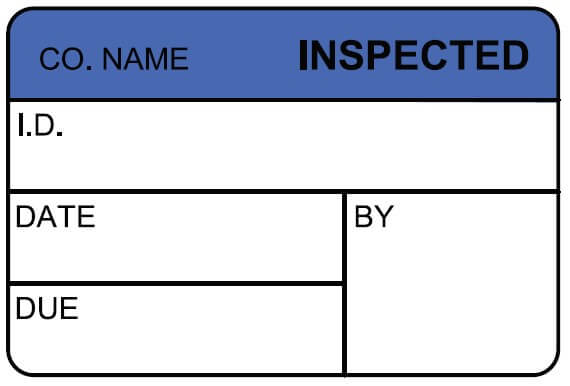
5 Label Requirements to Help Meet Certification Standards
To meet certification standards, labels and tags require direct traceability and must reference:
- The name of responsible body or organization using them.
- An I.D. number (i.e., serial number or other equipment identifier. This confirms certification for a specific piece of equipment, which ensures integrity and avoids misuse.
- The date of the equipment’s procedure (e.g., calibration) and the date when the next one is due to be performed.
- Who performed the procedure, with an area for an I.D. stamp.
- A part number that refers to that specific label or tag. This is to be included in your quality manual per ISO 9001 (4.2) ISO/IEC Guide 25 (5).
Understanding the difference between ISO/IEC GUIDE 25 and ISO 9000
The adopted worldwide standards are:
- ISO/IEC Guide 25 is for accrediting laboratories. Laboratory accreditation by an authoritative body gives formal recognition based on specific standards that measure its competence.
- ISO 9000 is for registering quality systems. Quality registration is certified by a third party and relies on recognition systems that give written assurance of conformance to specified requirements. The quality registration is defined in terms of a particular scope. It does not certify quality for compliance to specific technical specifications and requirements.
It’s worth noting that ISO/IEC Guide 25 establishes technical competence and includes the relevant ISO 9000 quality requirements, e.g., ISO 9002.
In itself, however, ISO 9002 does not satisfy the requirements or intent of ISO/IEC Guide25 because it does not address technical compliance. ISO/IEC Guide 25 alone, in contrast, is only a starting point for accreditation criteria. The methods and calibration procedures must be evaluated as well for a laboratory’s competence to be formally recognized.
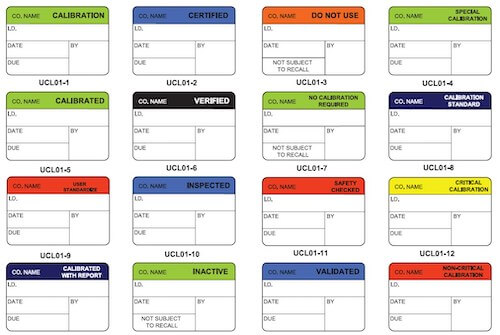
ID Label’s labels and tags allow you to meet requirements for virtually all international quality standards, including ISO, ANSI/ASQC and JCAHO.
Our Labeling Products Include:
- Custom label design and consultation at no extra charge
- 20-step quality assurance production process
- Digital production proofs
- Sequential number tracking and database management, if applicable
Our standard calibration labels are designed for GMP (Good Manufacturing Practices) and industry standards requirements for equipment calibration and identification.
Our labels and tags allow you to meet requirements for virtually all international quality standards, including ISO, ANSI/ASQC and JCAHO.
Request a Catalog and Label Samples
Our custom, durable labels will help you meet virtually any industry standard requirements, whether for quality control, equipment calibration, biomedical, industrial or engineering label applications. Request samples and a catalog to learn more!

The ID Label Advantage
ID Label produces millions of labels annually for major hospitals and healthcare networks, leading biomedical and clinical engineering organizations, world-renown educational institutions and leading pharmaceutical companies.
Our labels and tags can be produced in a wide variety of colors and formats to satisfy a broad range of applications. Request samples to learn more.
Interested in learning more? Contact us today.
